High-efficiency phosphopeptide and glycopeptide simultaneous enrichment
Time:2020-05-20 14:32 Author:
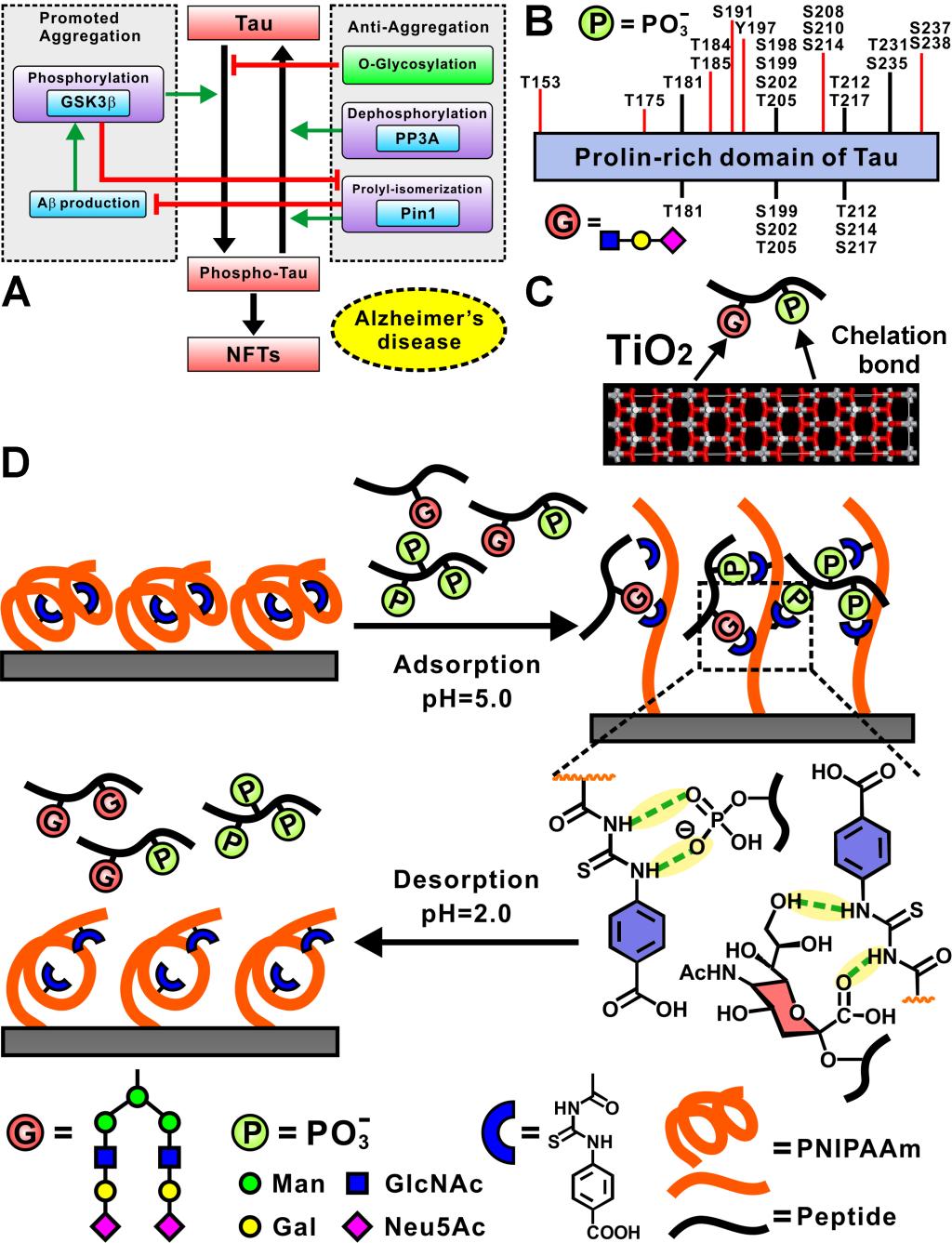
Aberrant protein phosphorylation and glycosylation are closely associated with a number of diseases. In particular, an interplay between phosphorylation and glycosylation regulates the hyperphosphorylation of protein tau, which is regarded as one of the pathologic features of Alzheimer’s disease (AD). However, simultaneous characterization of these two types of post-translational modifications (PTMs) in the complex biological samples is challenging. TiO2 and the immobilized ion affinity chromatography (IMAC)-based enrichment method suffers from low selectivity and/or low recovery of phosphopeptides and glycopeptides because of the inherent limitations in intermolecular interactions. Here, we introduce a hydrogen bond-based poly[(N-isopropylacrylamide-co-4-(3-acryloylthioureido)benzoic acid0.2] (referred to as PNI-co-ATBA0.2) as a bifunctional enrichment platform to solve this bottleneck problem. Benefited from multiple hydrogen bonding interactions of ATBA with N-acetylneuraminic acid (Neu5Ac) located at the terminals of sialylated glycans and from favorable conformational transition of the copolymer chains, the smart copolymer has high adsorption capacity (370 mg·g–1) and high recovery (ranging from 74.1%±7.0% to 106%±5.0% (n=3)) of sialylated glycopeptides. The smart copolymer also has high selectivity (79%) for simultaneous enrichment of glycopeptides and phosphopeptides from 50 mg HeLa cell lysates, yielding 721 unique phosphorylation sites from 631 phosphopeptides and 125 unique glycosylation sites from 120 glycopeptides. This study will open a new avenue and provide a novel insight for the design of enrichment materials used in PTM-proteomics.
High-efficiency phosphopeptide and glycopeptide simultaneous enrichment by hydrogen bond–based bi-functional smart polymer
Qi Lu, Cheng Chen, Yuting Xiong, Guodong Li, Xiaofei Zhang, Yahui Zhang, Dongdong Wang, Zhichao Zhu, Xiuling Li,* Guangyan Qing,* Taolei Sun, and Xinmiao Liang
Anal. Chem., 2020. 92, 6269
DOI: 10.1021/acs.analchem.9b02643