Recently, a research team of Prof. Qing, a group of bio-separation and interfacial molecular mechanism, and a team of Prof. Li, a laboratory of molecular modeling and desigh, designed and prepared a pair of chiral amino acid−modified phospholipids, and displayed the remarkable influence of molecular chirality of chiral liposomes on the amyloid formation.
Alzheimer's disease (AD), the most common form of dementia, is one of the biggest global public health challenges facing our generation. Up to now, a great majority of drugs end in failure, the root cause of this tragic result is the unclear pathogenesis. A large number of studies have shown that cell membranes play crucial roles in the progress of AD, particularly amyloid-β (Aβ) accumulation. Thus, investigating the effect of molecular interfaces, particularly biological membranes, on amyloid formation is essential. Most previous studies have reported the effect on amyloid formation of the properties of phospholipid membranes, such as their compositions, hydrophobicity‒hydrophilicity, charge, curvature, rafts and so no. However, researchers ignore an important issue, that is, the natural phospholipid is a chiral molecule showing very high preference to the L-enantiomer. More importantly, life is a typical chiral system and chiral phenomena are ubiquitous in nature from molecular, macromolecular, supramolecular to macroscopic levels. Countless biological and physiological processes are closely related to molecular chirality. This strongly inspires researchers to investigate how molecular chirality of the phospholipid membrane impact on Aβ aggregation process, and provides new ideas for studying the pathogenesis of AD.
Qing’s team first designed and prepared a pair of chiral amino acid−modified phospholipids with satisfactory mirror symmetry. Then, chiral phospholipids were processed and self-assembled into specific size chiral liposomes to simulate real cell membrane surfaces and co-incubate with Aβ. We found that the self-assembled L-liposomes slightly inhibited Aβ(1−40) nucleation process and could not affect oligomer elongation process. By comparison, the D-liposomes strongly inhibited both the nucleation and elongation processes of Aβ(1−40). Notably, chiral liposomes not only have good biocompatibility but also can rescue Aβ(1−40)‒aggregation induced cytotoxicity with significant chiral discrimination, in which the cell viability is higher in the presence of D-liposomes. Meanwhile, Li’s team clearly revealed the binding site, binding mannersand driving force between Aβ(1−40) and chiral phospholipid surfaces through detailed molecular dynamics simulations. This work largely expands the research from artificial chiral surfaces to real chiral phospholipid surfaces, providing a deeper and real insight to understand the crucial amyloidosis process from the perspective of chiral biointerface. Notably, liposomes have convincing biocompatibility and can be internalized by cells, the convergence of liposomes with non-natural D-amino acids as amyloid inhibitors have great application prospects in the AD early prevention and treatment, which points out a clear direction for the development of liposome‒based inhibitors.
Related achievement was published in Chemical Science (2020, DOI: 10.1039/D0SC02212H, https://pubs.rsc.org/en/content/articlelanding/2020/sc/d0sc02212h#!divAbstract). The first authors are Xue Wang and Cunli Wang from Qing's team, and Dr. Huiying Chu from Li's team. The above research work has been supported by the National Natural Science Foundation of China, the Start-up Fund of the Innovation Special Zone Group of DICP, and the Xingliao Elite Program. At the same time, the Key Laboratory of Separation and Analytical Chemistry, CAS, the Division of Energy Research Resources, and the DICP provided strong cultivation and support!
Molecular chirality mediated amyloid formation on phospholipid surfaces
Xue Wang, Cunli Wang, Huiying Chu, Haijuan Qin, Dongdong Wang, Feifei Xu, Xuanjun Ai, Chunshan Quan, Guohui Li* and Guangyan Qing*
Chem. Sci. 2020 DOI: 10.1039/D0SC02212H
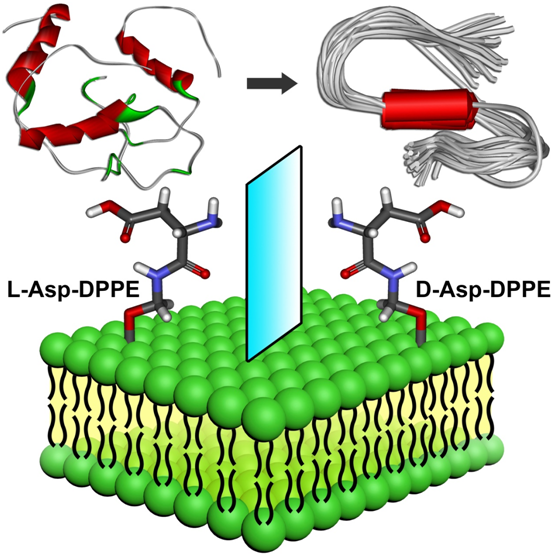
Remarkable inhibition effect and chiral discrimination are observed when the amyloid peptide aggregates on chiral phospholipid surfaces. L-Asp-DPPE liposomes slightly inhibit Aβ(1−40) nucleation process but cannot affect oligomer elongation process. By contrast, the D-Asp-DPPE liposomes strongly inhibit both nucleation and elongation of the peptide. Notably, L- and D-Asp-DPPE liposomes not only have good biocompatibility but also can rescue Aβ(1−40)‒aggregation induced cytotoxicity with significant chiral discrimination, in which the cell viability is higher in the presence of D-Asp-DPPE liposomes. This study provides deeper understanding of the crucial amyloidosis process from the perspective of chiral interface, while reveals that the convergence of D-amino acids with the liposomes might be a feasible route for AD prevention.