H2O2 and phosphorylated peptide dual-responsive nanochannel device
Time:2023-06-14 09:31 Author:Cunli Wang
Lang Peng, Cunli Wang, Qiwen Lin, Yongxin Chang, Xiaoyu Zhang, Jing Wang, Zan Li, Zhiying Yang, Wenjing Sun, Wenqi Lu, Dongdong Wang, and Guangyan Qing*
Anal. Chem. 2023, DOI: 10.1021/acs.analchem.3c01458
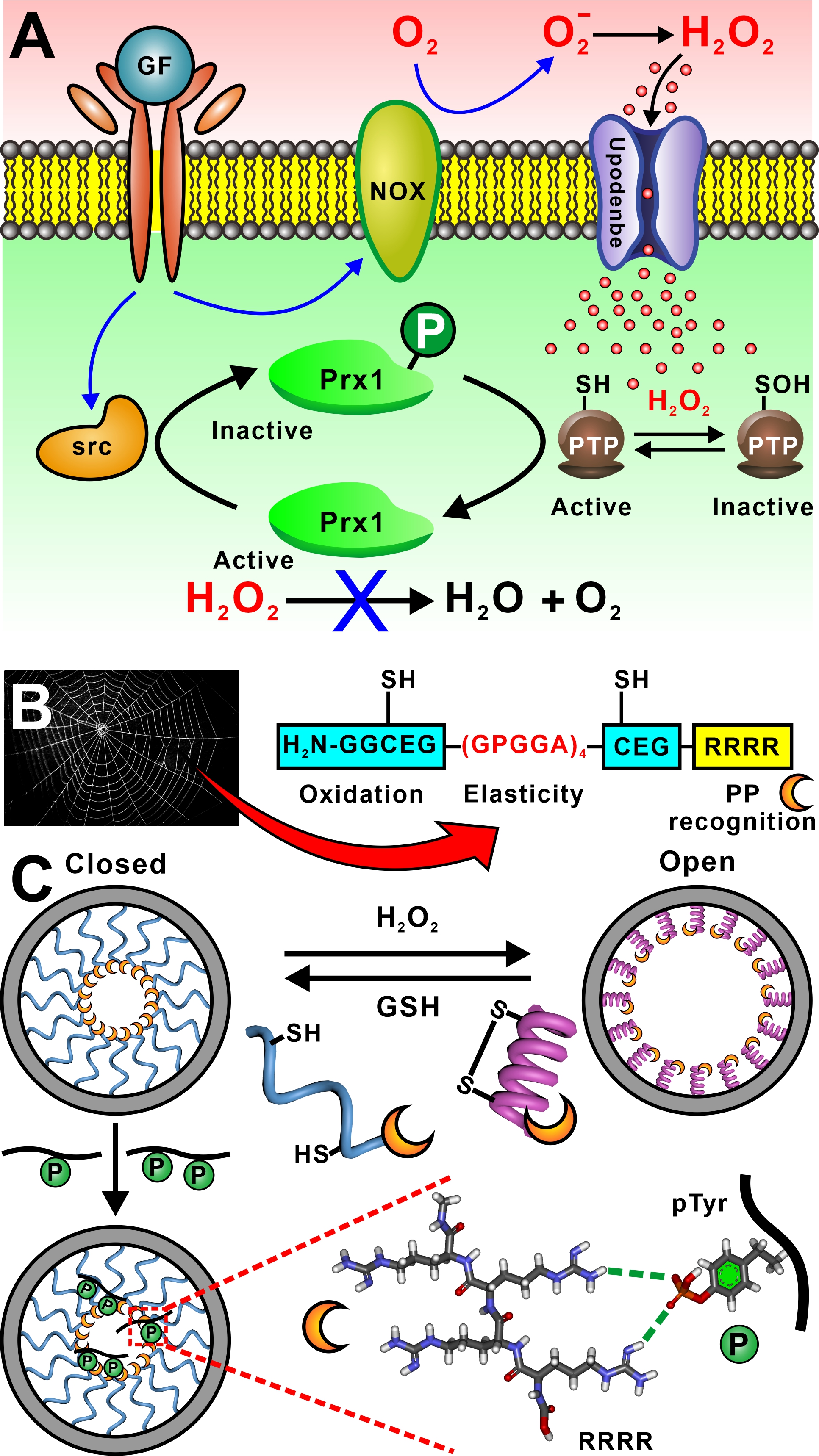
Oxidation and protein phosphorylation are critical mechanisms involved in regulating various cellular activities. Increasing research has suggested that oxidative stress could affect the activities of specific kinases or phosphatases, leading to alterations in the phosphorylation status of certain proteins. Ultimately these alterations can affect cellular signaling pathways and gene expression patterns. However, the relationship between oxidation and protein phosphorylation reminds complex and not yet fully understood. Therefore, the development of effective sensors capable of detecting both oxidation and protein phosphorylation simultaneously presents an ongoing challenge. To address this need, we introduce a proof-of-concept nanochannel device that is dual-responsive to both H2O2 and phosphorylated peptide (PP). Specifically, we design a peptide GGGCEG(GPGGA)4CEGRRRR, which contains an H2O2-senstive unit CEG, an elastic peptide fragment (GPGGA)4, and a phosphorylation site recognition fragment RRRR. When the peptides are immobilized on the inner walls of conical nanochannels in a polyethylene terephthalate membrane, this peptide-modified nanochannel device exhibits a sensitive response to both H2O2 and PPs. The peptide chains undergo a random coil to a-helix transition in response to H2O2, which leads to a close-to-open transition of the nanochannel, accompanied with remarkable increase in the transmembrane ionic current. In contrast, binding of the peptides with PPs shields the positive charge of the RRRR fragments, causing a decrease of the transmembrane ionic current. These unique features enable sensitive detection of reactive oxygen species released by 3T3-L1 cells stimulated by platelet-derived growth factor (PDGF), as well as the PDGF–induced change in PPs level. Real-time kinase activity monitoring further confirms the device’s potential utility for kinase inhibitor screening.