Identifying Umami Peptides Specific to the T1R1/T1R3 Receptor via Phage Display
Time:2023-08-01 09:48 Author:Mingyang Li
Mingyang Li, Xiaoyu Zhang, Yiwen Zhu, Xiancheng Zhang, Zhiyong Cui, Ninglong Zhang, Yue Sun, Zhiying Yang, Wenli Wang, Cunli Wang, Yin Zhang, Yuan Liu* and Guangyan Qing*
J. Agric. Food Chem. 2023, DOI: 10.1021/acs.jafc.3c02471
https://doi.org/10.1021/acs.jafc.3c02471
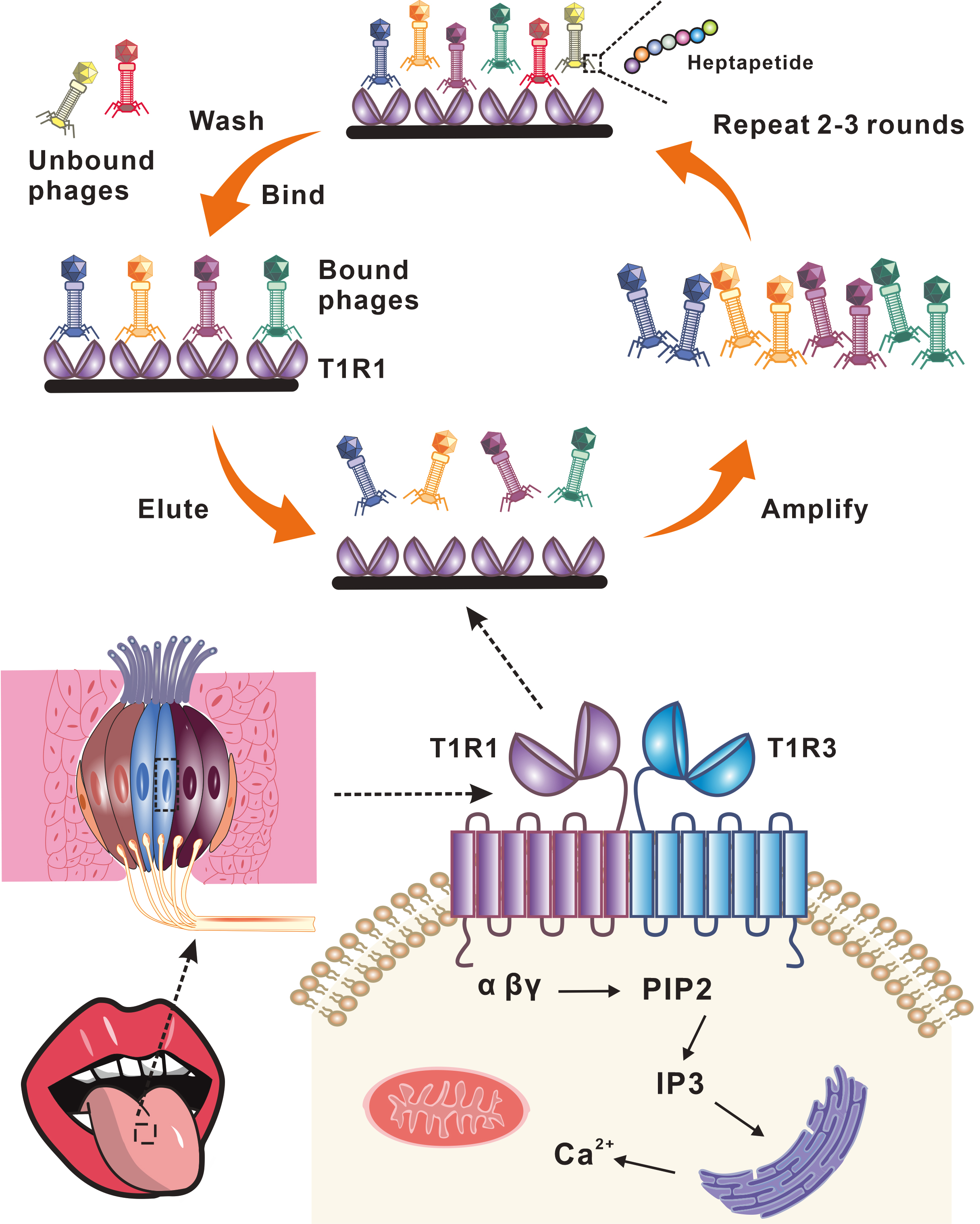
Umami peptides are small molecular weight oligopeptides that play a role in umami taste attributes. However, the identification of umami peptides is easily limited by environmental conditions, and the abundant source and high chromatographic separation efficiency remain difficult. Herein, we report a robust strategy based on a phage random linear heptapeptide library that targets the T1R1-Venus flytrap domain (T1R1-VFT). Two candidate peptides (MTLERPW and MNLHLSF) were readily identified with high affinity for T1R1-VFT binding (KD of MW-7 and MF-7 were 790 and 630 nM, respectively). The two peptides exhibited umami taste and significantly enhanced the umami intensity when added to the monosodium glutamate solution. Overall, this strategy shows that umami peptides could be developed via phage display technology for the first time. The phage display platform has a promising application to discover other taste peptides with affinity for taste receptors of interest and has more room for improvement in the future.