Yahui Zhang, Xiangyu Zhao, and Guangyan Qing*
Chemical Synthesis 2024, DOI: 10.20517/cs.2023.38
https://www.oaepublish.com/articles/cs.2023.38
Recently, our group systematically summarized the research status of electrochemical-induced hydrofunctionalizations of alkenes and alkynes, and discussed and described the current challenges and future developments.
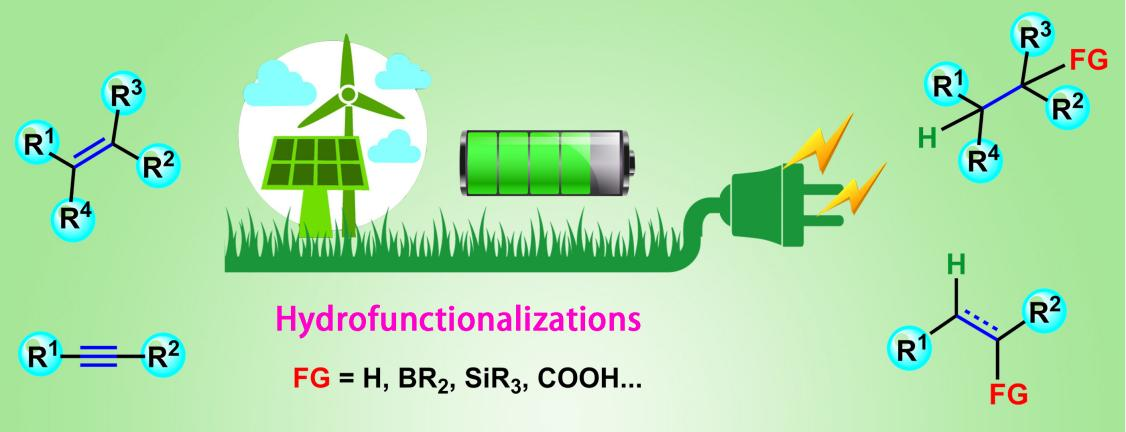
The hydrofunctionalizations of alkenes and alkynes is one of the most atomically economical ways to produce high value-added compounds. Compared with the traditional transition metal catalytic methods, the electroorganic synthesis strategy has the advantages of being simpler, gentler, and more economical. Alkenes and alkynes, as common synthetic blocks, are widely used in organic synthesis, agrochemicals, and bioorganic materials. Hydrofunctionalizations is an important type of reaction in organic synthesis. In the past few decades, various homogeneous and heterogeneous metal catalytic systems (including precious metals palladium, ruthenium, rhodium, iridium, and cheap metals copper, cobalt, iron, nickel, ect.) and non-metallic catalytic systems (such as Lewis acid, ionic liquids) have been developed and become powerful tools to achieve hydrofunctionalizations of unsaturated carbon–carbon bonds. Then various alkanes and alkenes with diversified structures were constructed. Although traditional catalytic systems play an important role in the hydrofunctionalizations of unsaturated bonds, there is an urgent need to develop greener and more sustainable organic synthesis methods driven by resource consumption, environmental crisis, and the dual carbon goal. Electroorganic synthesis is a new paradigm for energy transfer from green energy to high value-added chemicals, utilizing renewable electricity as an energy source, electrons as redox agents, and precisely controlling reaction selectivity and product yield parameters by adjusting current or voltage. Electrochemical-induced hydrofunctionalizations of alkenes and alkynes, which can avoid the addition of stoichiometric oxidants and reducing agents and the use of hydrogen under high pressure, has attracted widespread attention from scientists. In this paper, various electrochemical hydrofunctionalization reactions of alkenes and alkynes in recent years are summarized, and the research progress of hydrogenation of unsaturated carbon–carbon bonds and other hydrofunctionalization reactions (hydroboration, hydrosilylation, hydroalkylation, hydroalkoxylation, hydrocyanation, and hydrocarboxylation, etc.) are described in detail.

However, despite significant progress made in electroorganic synthesis, a series of issues still need to be addressed to promote the more efficient and selective electrochemical production of high value-added chemicals. This not only applied to the hydrofunctionalizations of alkenes and alkynes, but also provide prospects for the entire field of electrosynthesis research: (1) Electrochemical facilitated chiral synthesis is a new development trend, which requires the design and preparation of efficient electrochemical chiral catalysts; (2) The combination of electrical energy with other energy sources, including radio (Fig. 1a) and light (Fig. 1b), etc., to further optimize the electrochemical reaction conditions; (3) Develop new electrode materials, reaction media and electrolytes to promote the development of more green and efficient electroorganic synthesis and in-depth study of interface effects; (4) Design, amplification, and integration of an electrocatalytic organic synthesis reactor with high efficiency, high-performance, high-throughput, and low-energy consumption (Fig. 1c); (5) Strengthen the basic research of electroorganic synthesis and explore the regulation laws of electrochemical systems, and apply them to the large-scale preparation of products.