Wenqi Lu, Xinjia Zhao, Minmin Li, Yuting Li, Chen Zhang, Yuting Xiong, Jiaqi Li, Han Zhou, Xianlong Ye, Xiaonong Li, Jing Wang, Xinmiao Liang*, and Guangyan Qing*
ACS Nano 2024, DOI: 10.1021/acsnano.4c01571
https://pubs.acs.org/doi/10.1021/acsnano.4c01571
Recently, Qing Guangyan, a researcher from the biological separation and interface molecular mechanism research group (1824 groups) of our institute, and liangxinmiao, a researcher from the Chinese Medicine Science Research Center (2800 groups), have made new progress in the structural analysis of neutral glycans. Through the strategy of derivatization labeling of glycans and the mutation of nanopores, the structural analysis of glycans based on protein nanopores has been realized, and the interaction mechanism between glycan molecules and nanopores has been revealed.
So far, the surface of each cell of each species studied by researchers has been "covered" with a "glycan coating", and more than half of the proteins will be glycosylated. Therefore, glycans play an important role in the physiological and pathological processes of organisms. However, due to the structural heterogeneity of the glycan itself (including complex monosaccharide composition, sequence, connection type, branching structure, and isomeric stereochemistry, etc.), it is extremely difficult to accurately analyze the glycan structure. Moreover, most glycans are neutral in nature, with low ionization efficiency, and neutral glycans only have hydroxyl functional groups and lack of charge, which limits their ability to interact with nanopores and is difficult to be sensed by nanopores. Therefore, the analytical methods of neutral glycans are greatly limited, which hinders the development of glycomics, leading to the lack of more in-depth understanding of the biological functions of neutral glycans in organisms. Therefore, it is urgent to realize the fine structure analysis of neutral glycans, especially to develop an efficient and simple method to realize the structure analysis of neutral glycan isomers.
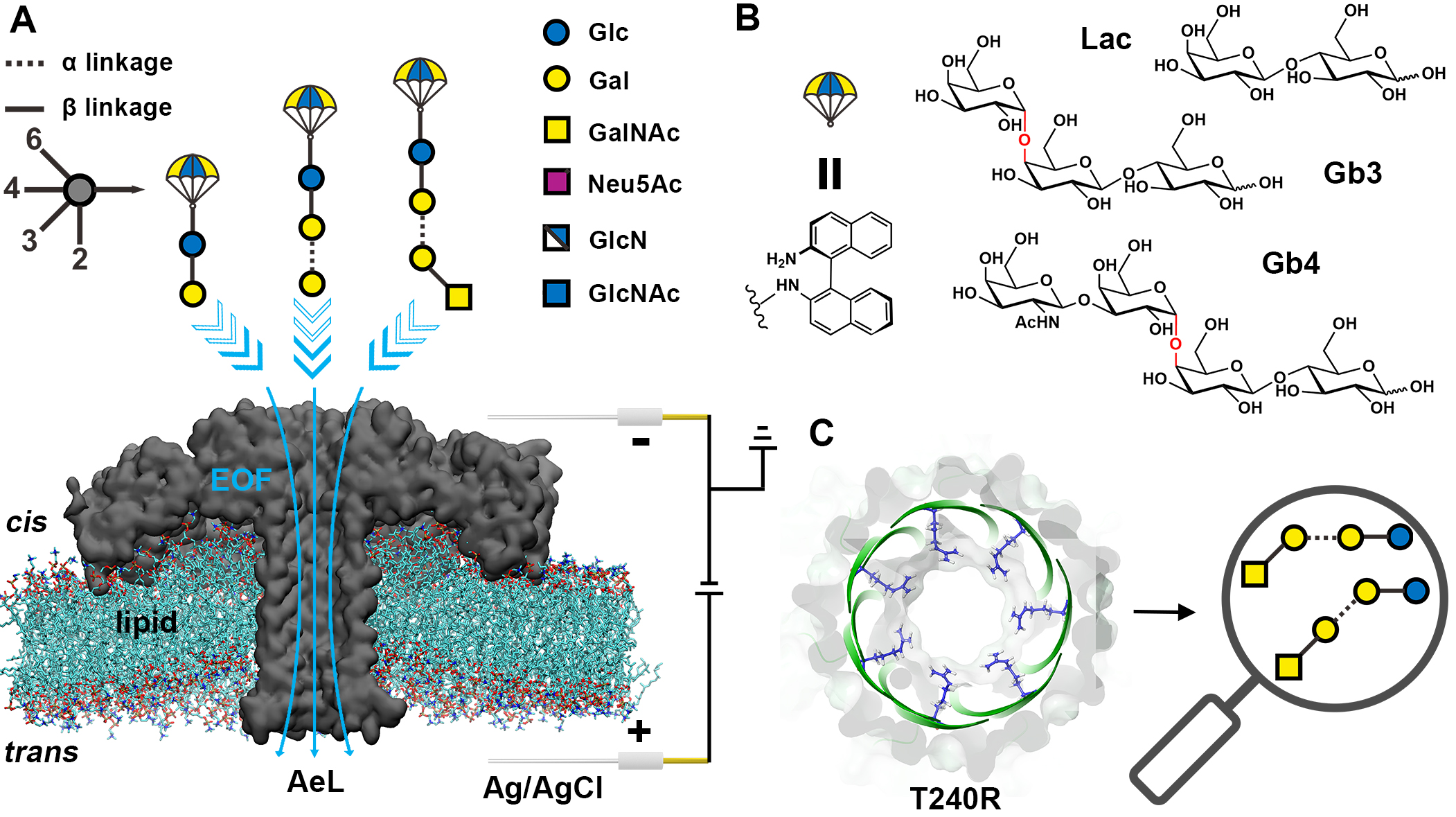
In the early stage, the cooperation team realized the differentiation of sialylated glycan isomers based on the solid-state nanopore sensing strategy of steady-state ion current (Chem. Sci. 2020 https://pubs.rsc.org/en/content/articlelanding/2020/SC/C9SC05319K )And nanopore sensing strategy based on transient ion current to realize the structural analysis of sialylated glycans (Nat. Commun. 2023 https://www.nature.com/articles/s41467-023-37348-5)Based on the above work, we designed and developed a glycan derivatization strategy with binaphthyl as a label, which enhanced the cation - π interaction between the derivatized glycan molecules and the nanopore interface, and slowed down the translocation speed of glycan molecules through the pores, so that we can detect neutral glycans using AEL nanopores. This method can distinguish different glycans by monosaccharide/group resolution, and has the potential to monitor enzymatic transglycosylation reaction. The mutant t240r obtained by mutation modification of AEL nanopores can identify six disaccharide isomers and trisaccharide and tetrasaccharide linked isomers. In addition, the collaborators of Ganjiang Science Center of traditional Chinese Medicine carried out machine learning and molecular docking, realized multi sample discrimination and mixed sample proportion prediction by using machine learning, and revealed the interaction mode between amino acid residues (R282, K238 and R240) on the inner wall of nanopores and glycan/binaphthyl tags through molecular docking simulation. In addition, more importantly, we demonstrated the kinetic translocation process of neutral glycan isomers for the first time, which laid a solid theoretical foundation for glycan analysis based on nanopores. The development of our technology can promote the analysis of glycan isomers, and pave the way for the determination and sequencing of glycan structure based on nanopores.
The relevant research, entitled "precise structural analysis of neutral glycans using aerolysin giant t240r nanopore", was recently published in ACS Nano. The first authors of this work are Yun Wenqi, a doctoral student from group 1824, and zhaoxinjia, an assistant researcher. The above work has been supported by the National Natural Science Foundation of China, the national key R&D program, the Institute's innovation fund and other projects.