Yongxin Chang, Juan Shao, Xinjia Zhao, Haijuan Qin, Yanqing Du, Junrong Li, Qiongya Li, Wenjing Sun, Guoxiong Wang, and Guangyan Qing*.
Advanced Science, 2024, 10.1002/advs.202405613.
https://onlinelibrary.wiley.com/doi/10.1002/advs.202405613
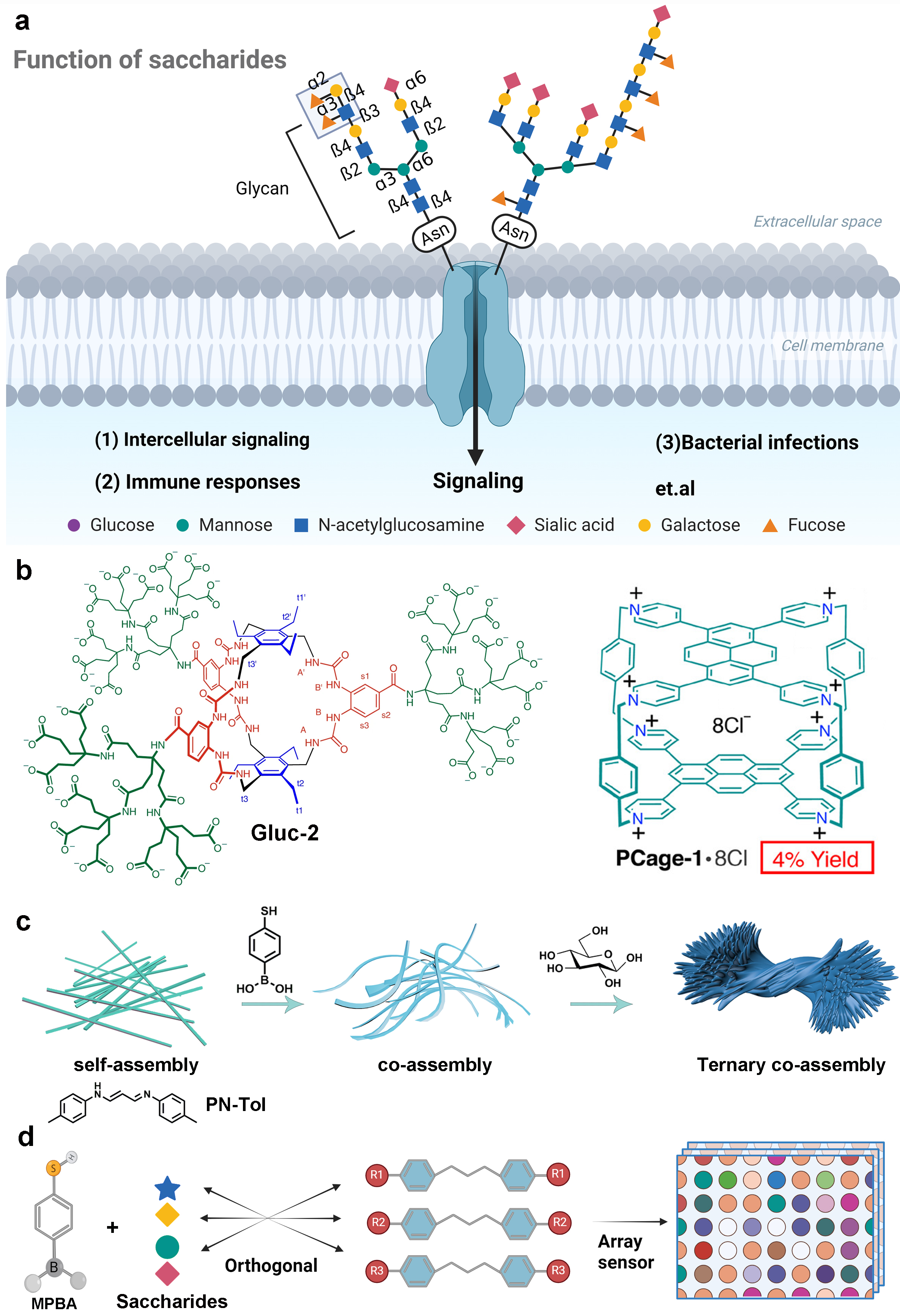
Saccharides are involved in nearly all life processes. However, due to the complexity and diversity of saccharide structures, their selective recognition is one of the most challenging tasks. Distinct from conventional receptor designs that rely on delicate and complicated molecular structures, here a novel and precise ternary co-assembled strategy is reported for achieving saccharide recognition, which originates from a halogen ions-driven aggregation-induced emission module called p-Toluidine, N, N′-1-propen-1-yl-3-ylidene hydrochloride (PN-Tol). It exhibits ultra-strong self-assembly capability and specifically binds to 4-mercaptophenylboronic acid (MPBA), forming highly ordered co-assemblies. Subsequent binding of various saccharides results in heterogeneous ternary assembly behaviors, generating cluster-like, spherical, and rod-like microstructures with well-defined crystalline patterns, accompanied by significant enhancement of fluorescence. Owing to the excellent expandability of the PN module, an array sensor is constructed that enables easy classification of diverse saccharides, including epimer and optical isomers. This strategy demonstrates wide applicability and paves a new avenue for saccharide recognition, analysis, and sequencing.