Smart Polymer–Based Calcium Ion Self-Regulated Nanochannel by Mimicking Biological Ca2+–induced Ca2+ Release Process.
Yunlong Li, Yuting Xiong, Dongdong Wang, Xiuling Li, Zhixiang Chen, Cunli Wang, Haijuan Qin, Jinxuan Liu, Baisong Chang, and Guangyan Qing*
NPG Asia Materials 2019, 11, 46.
DOI: 10.1038/s41427-019-0148-4
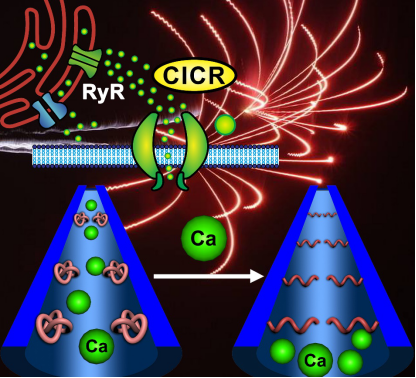
In nature, ion channels play key roles in controlling ion transport between cells and their surroundings. Calcium ion (Ca2+)–induced Ca2+ release (CICR), being a critical control mechanism for Ca2+ channels, occurs as a Ca2+ concentration gradient working in synergy with ryanodine receptors, which is famous as “calcium sparks”. Inspired by this self-regulated biological process, a smart Ca2+ concentration–modulated nanochannel system was developed by integrating a poly{N-isopropylacrylamide-co-acrylamide-[4-(trifluoromethyl) phenyl]-2-thiourea0.2-co-acrylamide-DDDEEKC0.2} (denoted as PNI-co-CF3-PT0.2-co-DDDEEKC0.2) three-component copolymer onto nanochannels of porous anodic alumina (PAA) membrane. In this smart polymer design, DDDEEKC hepta-peptide unit has an extraordinary binding affinity with Ca2+ through coordination bonds, while CF3-PT functions as a hydrogen bond mediation unit, facilitating a remarkable conformational transition of the PNI main chain in response to the Ca2+ specific adsorption. Due to these futures, dynamic gating behaviors of the modified nanochannels could be precisely manipulated by Ca2+ concentration. In addition, sensitive Ca2+ responsiveness as low as 10 pM, high specificity toward Ca2+ capable of discriminating Ca2+ from other potential interference metal ions (e.g., K+, Cu2+, Mg2+, Zn2+, Fe3+, and Al3+), remarkable morphological change in the nanochannel as well as satisfactory reversibility, indicates the great potential of Ca2+–responsive polymers in the fabrication of bio-devices and artificial nanochannels.