Recently, a research team of Prof. Qing, a group of bio-separation and interfacial molecular mechanism, and a team of Prof. Liang of the separation material chemistry and component Chinese medicine research, proposed a brand new strategy to capture of sialylated glycan (SG) based precisely, based on a dynamic covalent chemical method of Schiff base hydrolysis.
The glycans and glycoconjugates have a rich distribution on the surface of the cell membrane, like a layer of "glycocalyx" covering the cell surface. This outermost position and the wide distribution make SGs play a vital role in numerous physiological processes, such as viral infection, immune activation, cancer occurrence, development and migration. Therefore, sialylated glycoproteins have been widely used as clinical biomarkers for cancer. However, the identification and analysis of the SGs is extremely complex, and a new and efficient material system is urgently needed to overcome the bottleneck of sialylated glycopeptide enrichment and separation in complex biological samples and promote the development of comprehensive glycoproteomics.
The team first reported a strategy based on the dynamic covalent chemistry for the precise capture of SGs. This strategy is completely different from the traditional static affinity strategy, cleverly using the dynamic covalent chemical bonds present in Schiff base, and its hydrolyzed part forms a stable complex with the SG. The developed material exhibited excellent enrichment selectivity, high adsorption capacity and enrichment recovery rate toward the sialylated glycopeptides, reaching the highest level in the SG enrichment field. This provides biochemists with a novel, powerful, and landmark strategy that can be used to accurately capture glycan information that is closely related to the occurrence of cancer and immune diseases. On the other hand, the Schiff base material is easy to hydrolyze, completely subverting the knowledge that traditional enrichment materials must have good chemical stability. More importantly, the researchers found an exciting story behind the Schiff base hydrolysis reaction, demonstrating the unique advantages of dynamic covalent chemistry in the fields of glycoproteomics and biomolecular sensing.
Related achievements were published in the Journal of the American Chemical Society (2020, DOI: 10.1021/jacs.0c01970, https://pubs.acs.org/doi/10.1021/jacs.0c01970). The first authors are Dr. Yuting Xiong, a postdoctoral fellow in the Qing's group, and Dr. Xiuling Li, a researcher in the Liang's group. The above research work has been supported by the National Natural Science Foundation of China, the Start-up Fund of the Innovation Special Zone Group of DICP, and the Xingliao Elite Program. At the same time, Prof. Yonggui Zhou and Dr. Song Shi gave useful guidance to the work, and the Key Laboratory of Separation and Analytical Chemistry, CAS, the Division of Energy Research Resources, and the DICP provided strong cultivation and support!
What is hidden behind Schiff base hydrolysis? Dynamic covalent chemistry for precise capture of sialylated glycans
Yuting Xiong, Xiuling Li, Minmin Li, Haijuan Qin, Cheng Chen, Dongdong Wang, Xue Wang, Xintong Zheng, Yunhai Liu, Xinmiao Liang,* Guangyan Qing*
J. Am. Chem. Soc. 2020, 142, 7627
DOI: 10.1021/jacs.0c01970
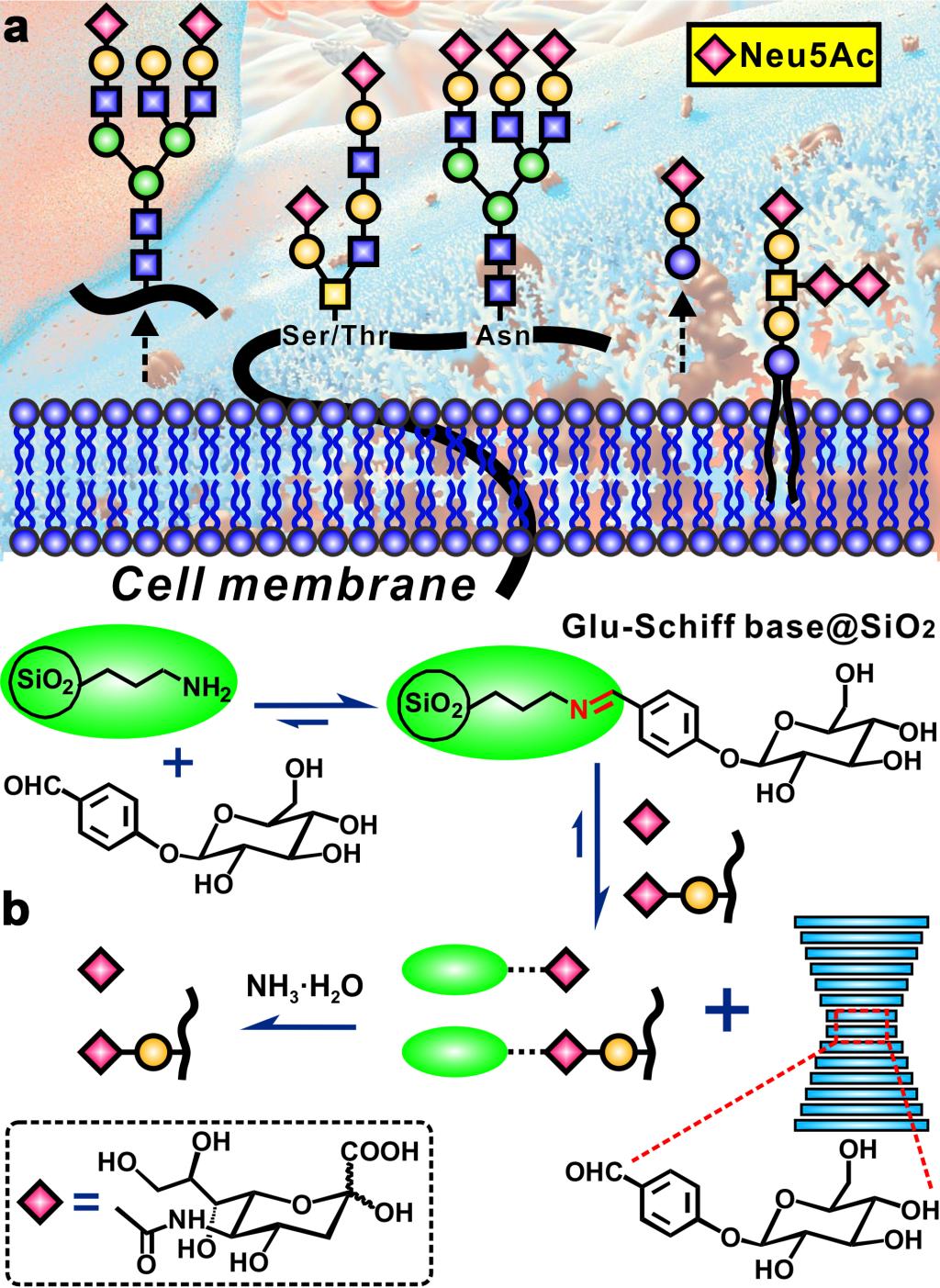
The aberrant expression of sialylated glycans (SGs) is closely associated with the occurrence, progression, and metastasis of various cancers, and sialylated glycoproteins have been widely used as clinical biomarkers for cancers. However, identification and comprehensive analysis of SGs is exceptionally complex, which urgently need innovative and effective method to capture SGs from biosample in prior to MS analysis. Here, we report that a novel dynamic covalent chemistry strategy based on Schiff base hydrolysis can be applied for the precise capture of the SGs. The prepared Glucopyranoside-Schiff base-modified silica gel displays extraordinary enrichment selectivity (even at the ratio of 1:5000 with interference), high adsorption capacity (120 mg·g−1), and satisfying enrichment recovery (95.5 %) towards sialylated glycopeptides, which contributes to a highly specific, efficient, mild and reversible SG capturing approach that can remarkably promote the development of glycoproteomics and sialic acid sensing devices, and can be considerably promising in cancer biomarker discovery. Meanwhile, the facile hydrolysis characteristic of our Schiff base material completely subverts conventional knowledge of enrichment materials, the chemical stability of which is usually regarded as a prerequisite. Importantly, we find an exciting story hidden behind the Schiff base hydrolysis reaction, which demonstrates the unique advantage of dynamic covalent chemistry in glycoproteomics and biomolecule sensing.