Tyrosine phosphorylation (pTyr) initiates cellular signaling, governs cellular functions, and its dysregulation is implicated in many diseases, especially cancers. pTyr-specific sensing is of great significance for developing targeted anti-cancer drugs. The commonly-used approach for detecting pTyr relies on the radiometric assay through employing [γ-32P]-ATP as a substrate. However, this approach suffered from the use of harmful radioactive reagent and generation of radioactive waste. Alternatively, antibody-based methods and some many synthetic chemical sensors have also been developed to achieve specific detection of pTyr for kinase inhibitor screening assays. However, they also suffered from the high cost arising from the use of specific anti-pTyr antibodies and the complicated labelling steps, respectively.
Recently, our group developed a new approach based on a functional ion nanochannel platform, and achieved the specific sensing of tyrosine phosphorylation. The results were published in J. Am. Chem. Soc. 2020, 142, 16324(DOI: 10.1021/jacs.0c06510)on September 7.
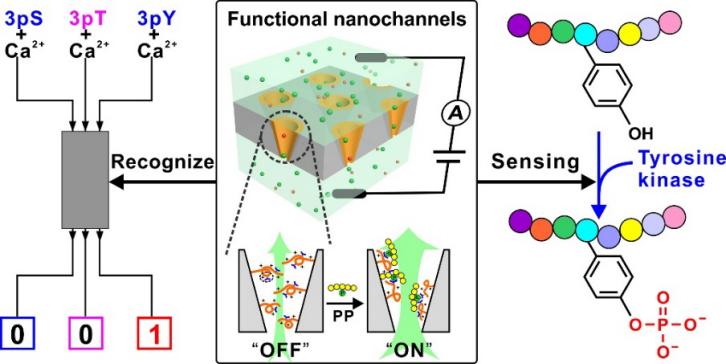
By mimicking the multiple interactions of guanidinium groups from arginine residues with phosphorylated residue in proteins, our designed a functional polymer bearing rich guanidinium groups to modified an ion nanochannel substrate, and developed a functional nanochannel device. The polymer could recognize the phosphorylated peptide (PP) through the binding of guanidinium group with phosphate group in PP, and could amplify such recognition to the conformational change of the polymer itself. Further, the conformational change was converted into the “OFF-ON” change of nanochannel ion flux, finally achieving the detection of PP by means of the change in ionic current.
The specific recognition for pTyr peptide from its counterparts pSer and pThr peptides was achieved by constructing a simple nanofluidic logic gate when Ca2+ was introduced as a competitive binding element. Importantly, the excellent pTyr sensing capacity make the functional nanochannels available for real-time monitoring of pTyr process by tyrosine kinase on a peptide substrate, even in a complicated condition, and the proof-of-concept study of monitoring kinase activity demonstrates its potential in kinase inhibitor screening.
This work was supported by the National Natural Science Foundation of China, DICP Innovation Funding and LiaoNing Revitalization Talents Program.