Label-Free, Versatile, Real-Time, and High-Throughput Monitoring of Tyrosine Phosphorylation Based on Reversible Configuration Freeze
Time:2023-04-26 19:34 Author:Yongxin Chang
Yongxin Chang, Miao Guo, Mengyuan Song, Wenjing Sun, Dongdong Wang, Minmin Li, Jixia Wang, Yahui Zhang, Haijuan Qin and Guangyan Qing*
CCS Chem. 2022, DOI: 10.31635/ccschem.022.202202070
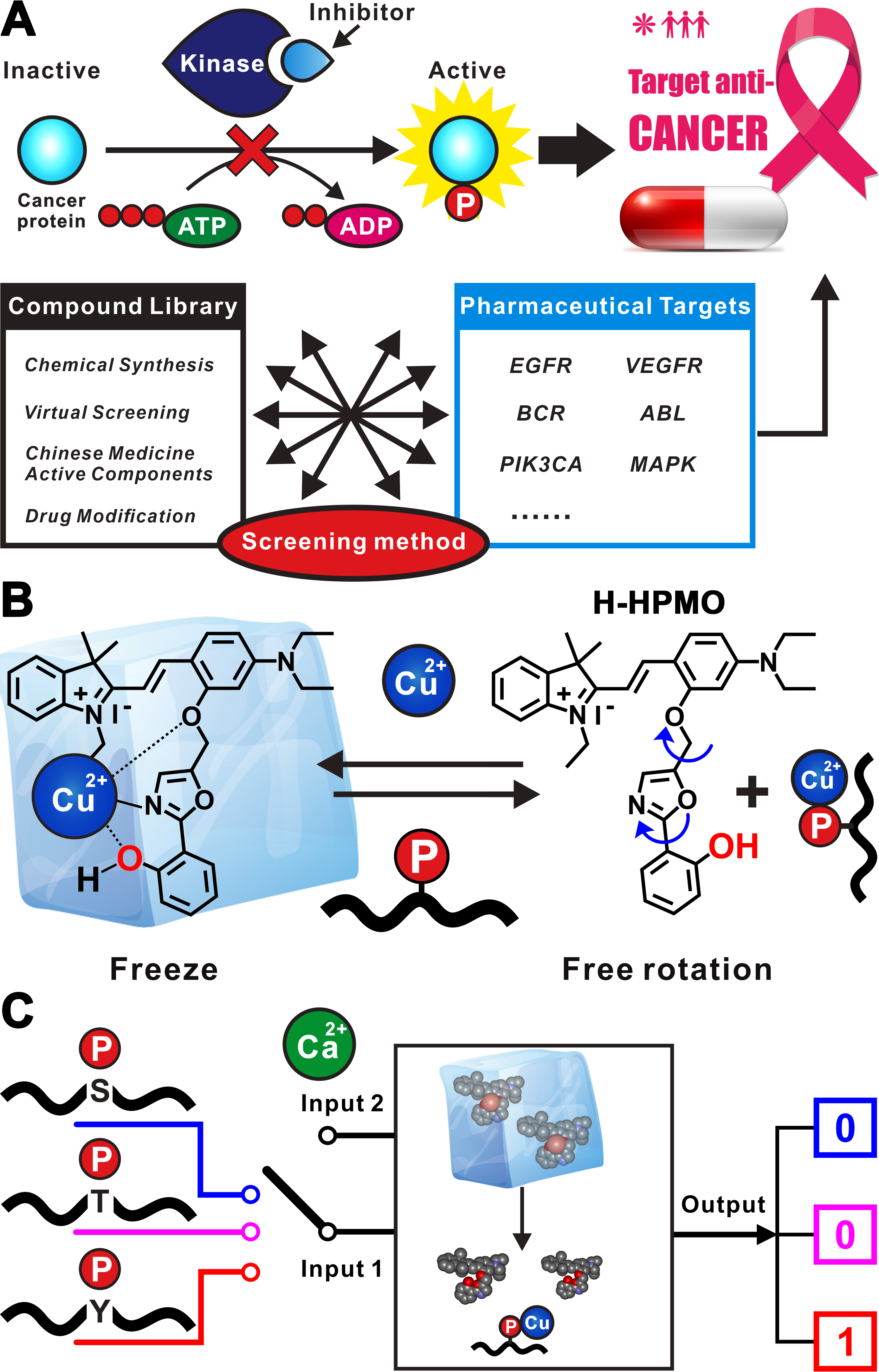
Tyrosine Phosphorylation (pTyr) is a critical and ubiquitous regulation mechanism in biology that plays a central role in controlling intracellular signaling networks. Precise recognition and specific detection of pTyr peptides have been of great importance for both discoveries of disease biomarkers and screening of therapeutic drugs, especially cancers. Here we report a label-free, versatile, realtime, and high-throughput detection strategy for phosphopeptide (PP) based on reversible configuration freeze of a unique hemicyanine-labeled 2-(2′-hydroxyphenyl)-4-methyloxazole (H-HPMO). By taking advantage of the “OFF–ON” transition of fluorescence, H-HPMO–Cu2+ complex displays a highly sensitive and selective response to PPs with modified sites on serine, threonine, and tyrosine. Specific recognition of Tyr PPs is achieved by performing a simple logic gate operation and introducing Ca2+ interference as an input. This PP detection approach is universal for various peptide sequences and displays high potential in large-scale kinase inhibitor screening, which will promote the development of targeted anticancer drugs.